-
 Welcome ToSynapse labs.com
Welcome ToSynapse labs.com -
 COMPETITIVE PRICING,TIMELY DELIVERY,QUALITY EXCELLENCE
COMPETITIVE PRICING,TIMELY DELIVERY,QUALITY EXCELLENCE -
-
-

-

-
-
-


Welcome to Synapse
Synapse Labs is a DCGI approved CRO(Contract Research Organization) based in Pune, India
Synapse Labs is a leading, independent Contract Research Organization (CRO) founded in 2007. We are offering comprehensive Bioavailability / Bioequivalence (BA/BE),Clinical Trial service,Pharmacovigilance and Regualtory Services to global pharmaceutical and biotechnology industry.
While leveraging on state-of-the-art facilities, we provide high quality, customized clinical research services with rapid turnaround time and at competitive price. Our experienced and GCP trained professionals contribute to the well-being of humanity by delivering quality research services in the process of drug development.
Read More
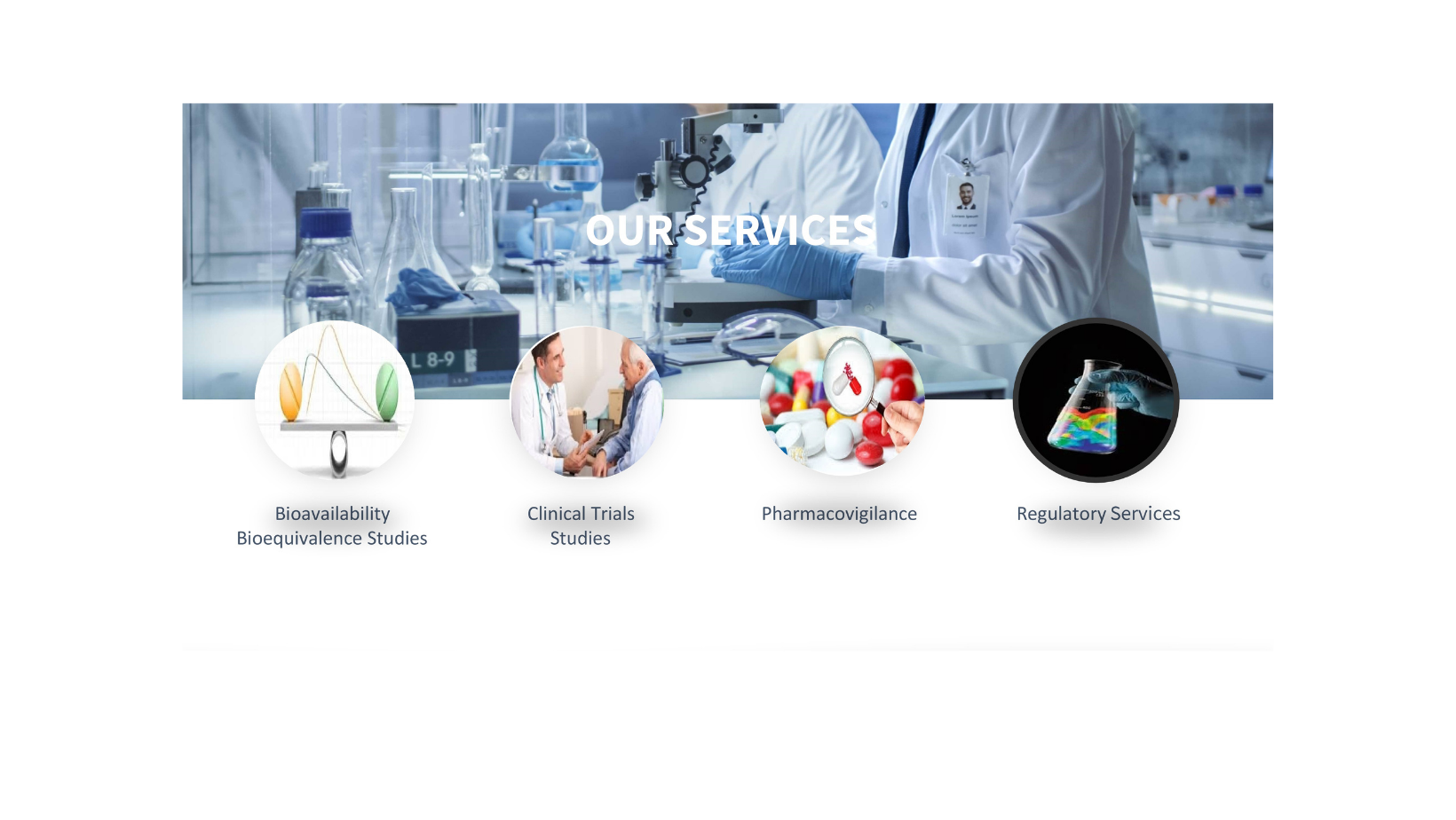
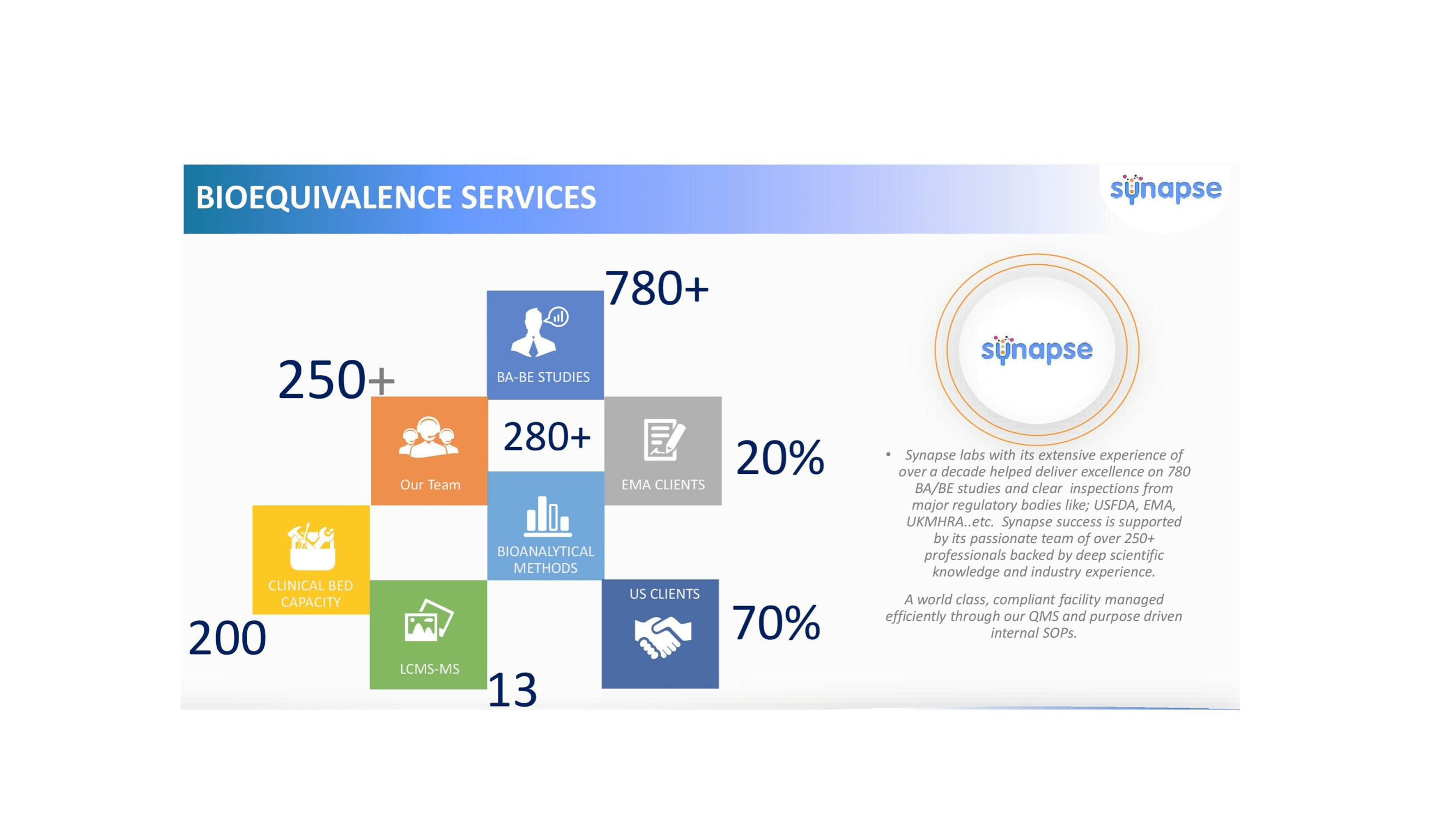
Bioavailability /Bioequivalence Services.
Solutions for remedial proportionally between an pharmaceutical comparable product
Clinical & Experimental Pharmacology
Chemical Specifications and Bioavailablity
Bioanalytical Methods Development & Bioequivalence Studies



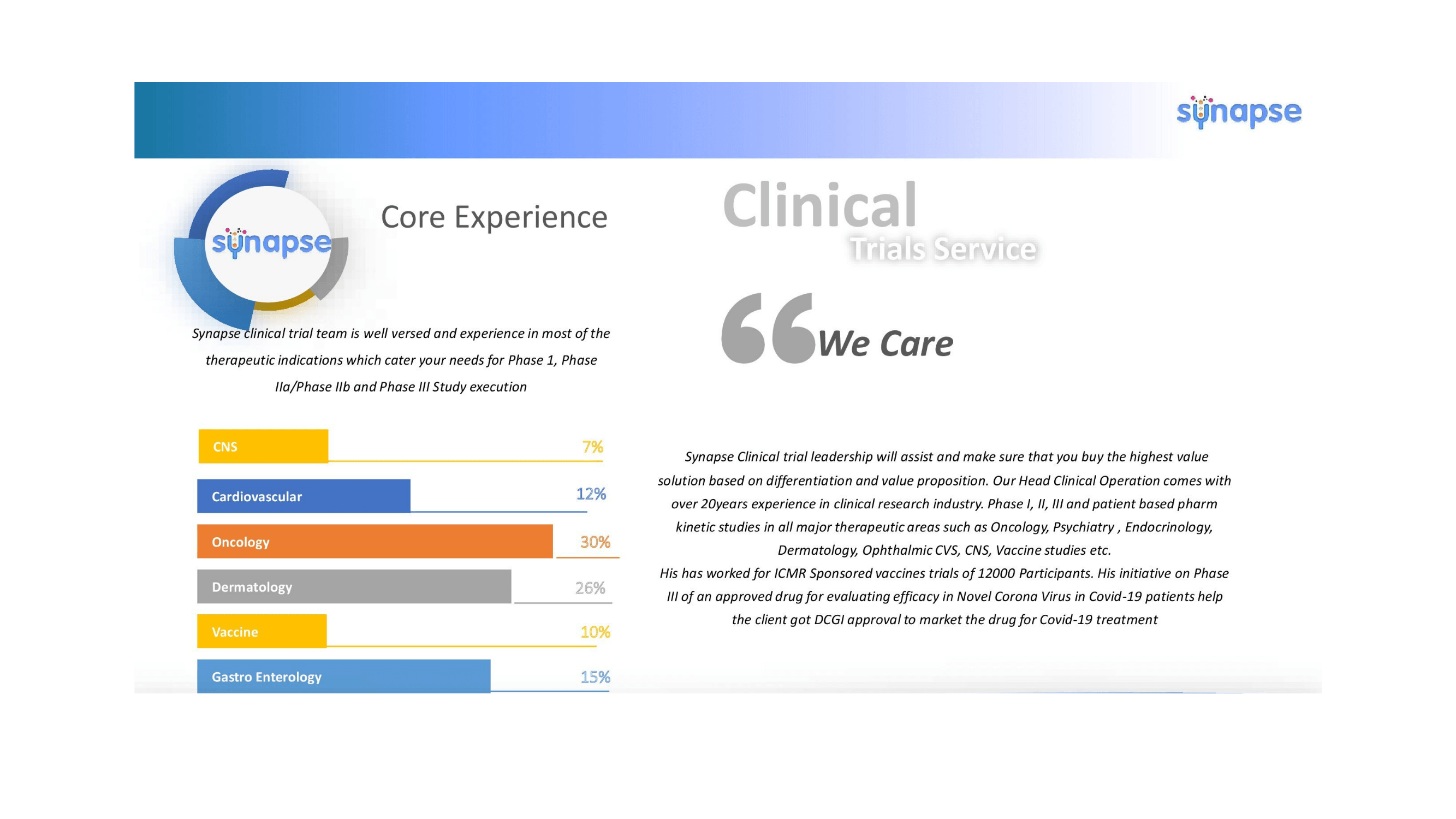
Clinical Trial Services
Experience on Major Therapeutic Indications.
Bioequivalence with Pk and Clinical End point Studies
Phase I/IIa/IIb/III/IV Trials
Database Mangement
NCE Molecule-IND, 505b(2) , ANDA Study Management.
FDA/PreIND MeetingSupport.

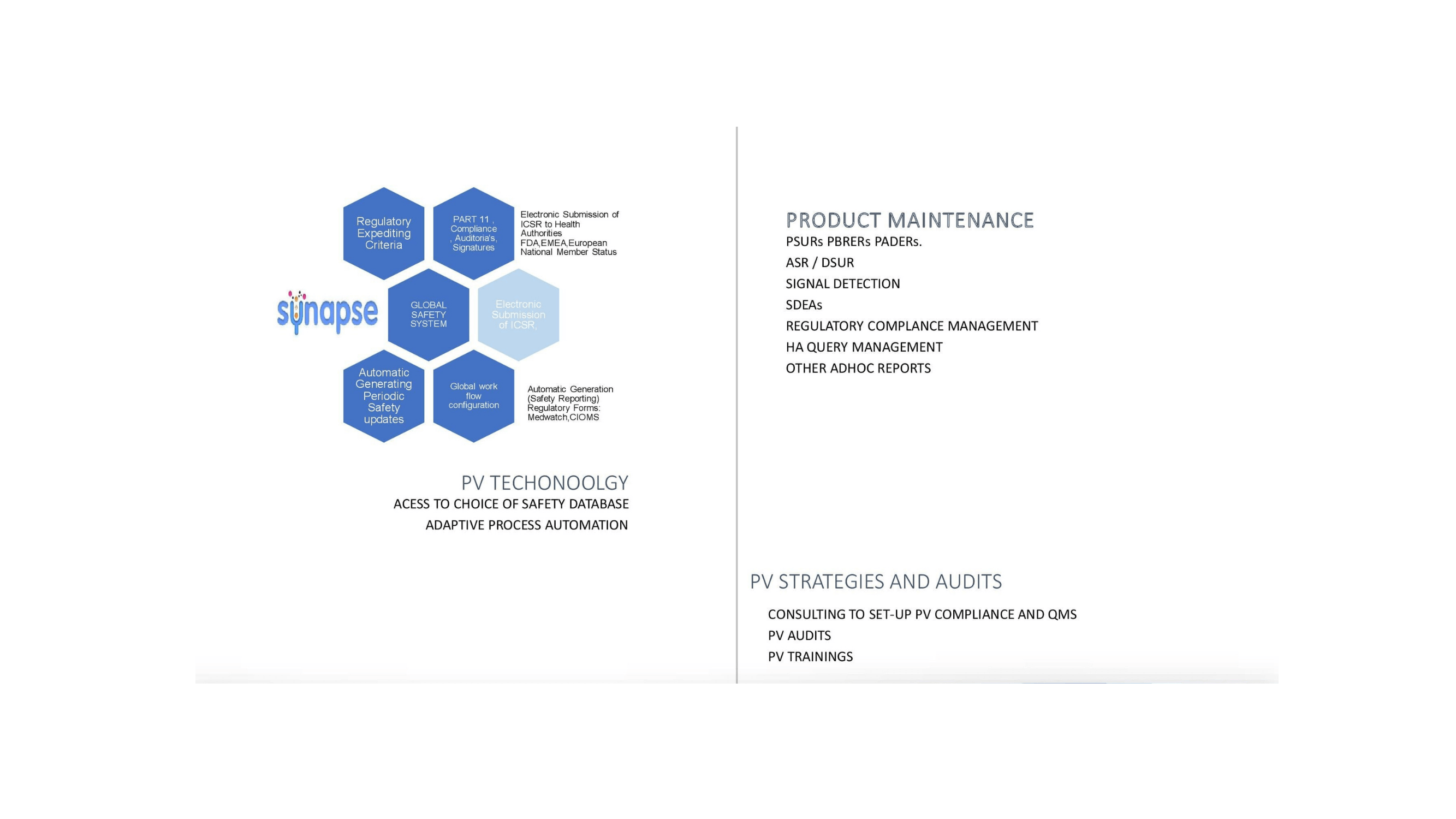
Pharmacovigilance Services
Safety Management
Clinical Pharmacovigilance, PMS Pharmacovigilance, Literature Automation/Literature Search, Aggregate/ Periodic Reporting, Signal Detection, Risk Management, Safety Data Exchange Agreements.
Medical Information Center
Medical Information/Database centers, Medical Enquires, Product Quality complaints, Standard /Customized Response Letters.
Medical Writing
Aggregate/Benefits/Risk Reports, Risk Management Plans, Safety Update Reports, Annual Updates, Medication Guides, Patient information leaflets
Automation and Technologies
Safety System Separate Database Environment, Electronic Submission , Automated Signal Detection System, Electronic Document Management, Data Migration.



Regulatory Services
CDSCO Application/ Approvals for Invitro/Invivo study conductions
Dossier Gap Analysis, Complete Dossier Writing, Dossier Support Services Translations (Smpc, PIL & Labelling), PIL User Testing, QP Audits, e-CTD Publishing, DMF, Dossier Maintenance and Labelling Maintenance,Dossier Variation Management.
Compliance on Warning Letters, Clinical Study Monitoring, GCP-GLP Audit Support.
Module e-CTD Compilations: Module 1- Module5, Complete Dossier Preparation
PreIND Meeting/ FDA Control correspondence
Regulatory Inspections/ Audits














